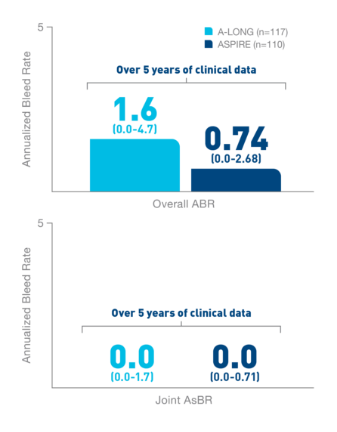
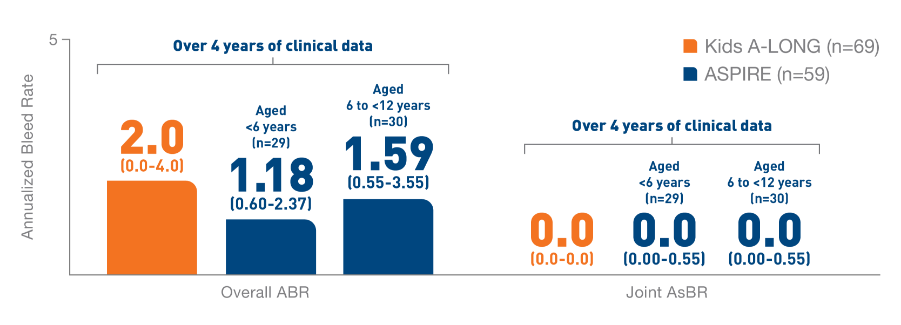
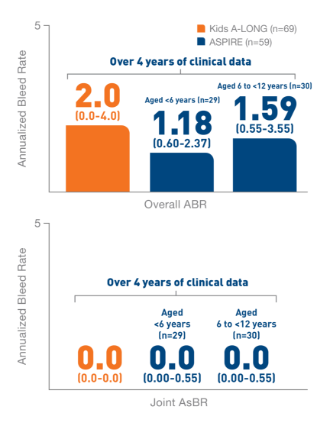
*ELOCTATE
has been proven to help
patients prevent bleeding episodes using a prophylaxis
regimen.1
In the Kids A-LONG
study, individualized prophylaxis regimen: 25 IU/kg
and 50 IU/kg of ELOCTATE on the first and fourth
days of the week, respectively. Adjustments in dose
(25 to 80 IU/kg) and interval (every 2 days or
longer) were allowed based on a subject’s available
PK data and observed bleeding pattern.1
In
the ASPIRE study, the individualized prophylaxis
regimen was 25 to 65 IU/kg every 3 to 5 days, or
twice weekly (20 to 65 IU/kg on Day 1, 40 to 65
IU/kg on Day 4). In subjects <12 years of age,
dose adjustments were made up to a maximum of 80
IU/kg up to every 2 days, if necessary.7
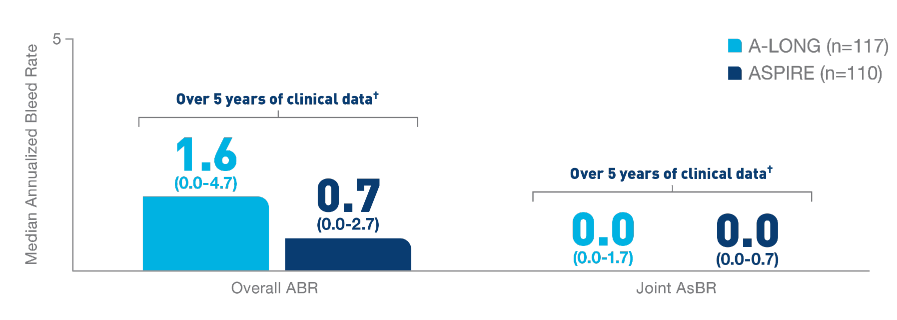
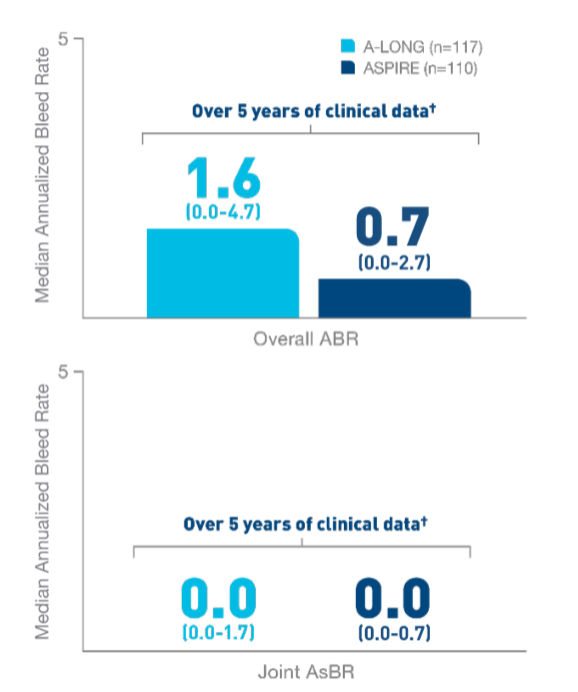
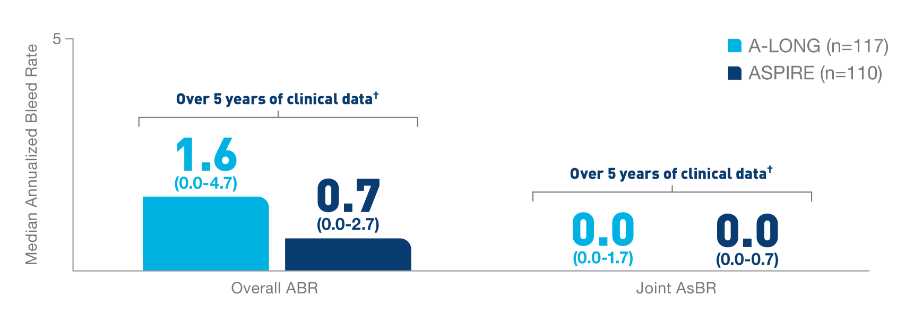
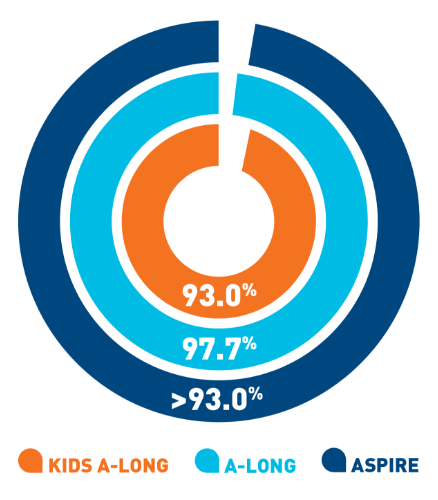
Nearly 100% of
target joints‡ were resolved with
ELOCTATE
prophylaxis6§
- In 111 adult and adolescent patients taking
ELOCTATE prophylaxis, 234 out of 235 target
joints were resolved
- In 13 pediatric patients taking ELOCTATE
prophylaxis, 9 out of 9 target joints were
resolved
‡A target joint is
defined as a major joint with ≥3 bleeding episodes
in a consecutive 6-month period. Target joint
resolution is defined as ≤2 spontaneous bleeds in a
12-month period.6
§Data are from the
third interim cut of ASPIRE taken on January 11,
2016. Forty-eight adult and adolescent patients and
seven
pediatric patients had no target joint bleeding
episodes.
![ELOCTATE® [Antihemophilic factor (recombinant), Fc fusion protein]](../assets/media/header/logo-eloctate.png)